
Request a pharmacist pack
- Dispensing and supplying therapeutic vapes – 16-page booklet
- Checklists: 'Supplying S3' and 'Dispensing S4'
- Nicovape® Q product sheet
- 50 x Patient enquiry hand-outs
- 50 x New patient guide hand-outs
Individual resources

Dispensing and supplying therapeutic vapes – 16-page booklet
A comprehensive summary of the practical aspects of the PSA Guidelines and TGA requirements for supplying and dispensing therapeutic vaping products

Patient enquiry hand-out
A handy 2-sided DL explainer to assist you with ongoing patient enquiries – 'What you need to know about therapeutic vaping'

Nicovape® Q new patient guide hand-out
Everything your patients need to know about setting up their new Nicovape® Q, initial and ongoing use, maintenance, and troubleshooting
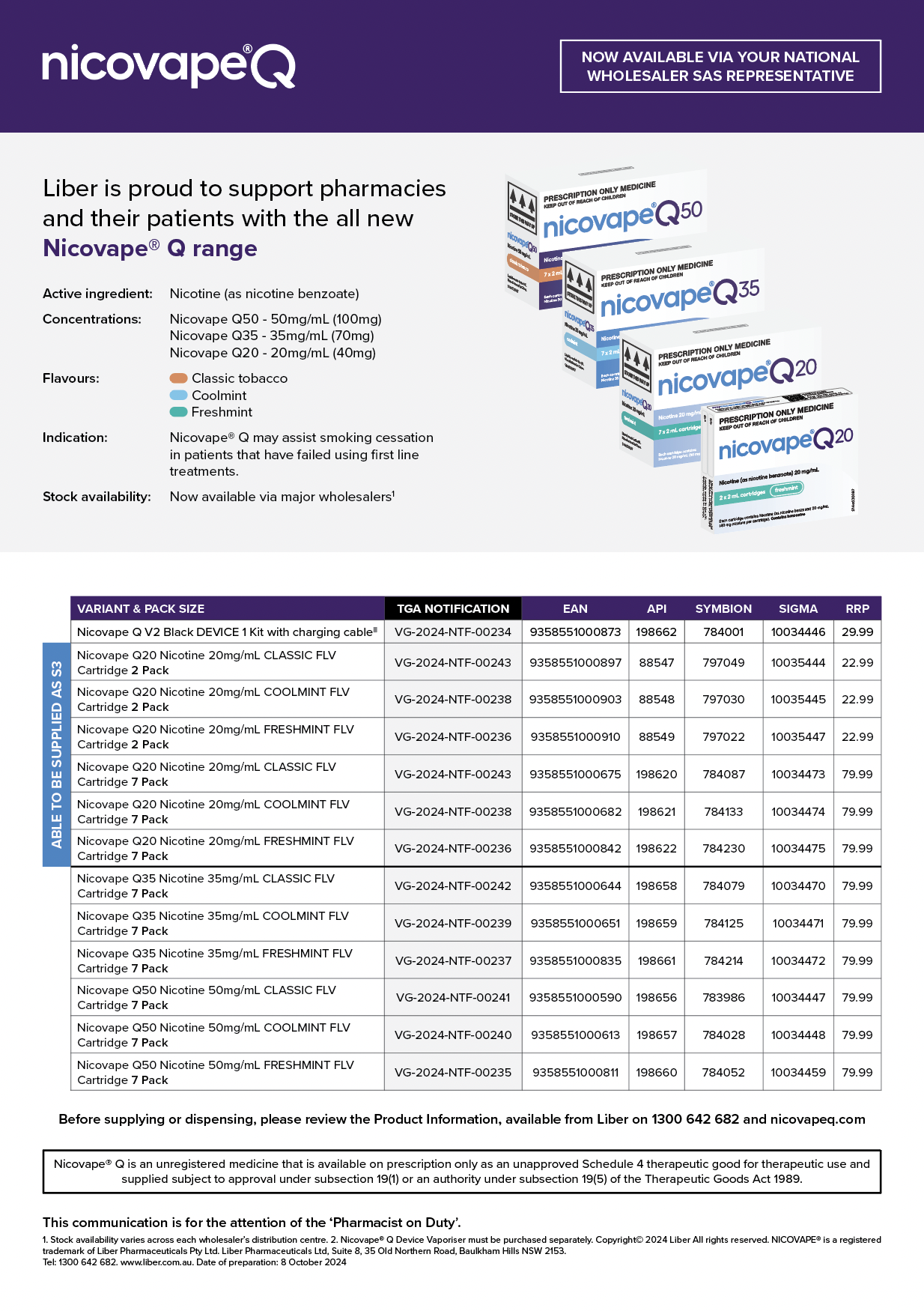
Nicovape® Q product sheet
The Nicovape® Q product sheet includes product information and ordering codes for Symbion, Sigma and API

Checklist: Supplying S3 vapes
Step-by-step checklist to ensure compliance and clinical best practices when supplying S3 therapeutic vaping products

Checklist: Dispensing S4 vapes
Step-by-step checklist to ensure compliance and clinical best practices when dispensing S4 therapeutic vapes

Supplying Nicovape® Q20 as a pharmacist-only medicine
Practical guidance for pharmacists when a patient presents seeking access to low-concentration therapeutic vaping products

Nicovape® Q system compatibility
A simple 1-page document highlighting the differences between the Q1 and Q2 versions of the Nicovape® Q products

No-liquid Lock System (NLS) – troubleshooting guide
Nicovape® Q product update: Guidance on addressing patient queries relating to the No-liquid Lock System (NLS) feature of the Nicovape Q
Liber:Onboard
Onboard your pharmacy with Liber’s medical liaison team today
Liber:Activate
Establish your local GPs as activated authorised prescribers
Important links
Therapeutic Goods Administration
Pharmaceutical Society of Australia
© 2024 Liber Pharmaceuticals Ltd. All content
iNRT Healthcare is operated by Liber Pharmaceuticals for the purpose of furthering healthcare education regarding Nicotine Vaping Products (NVPs) within the Australian medical framework. Liber has no affiliation with the tobacco industry, their affiliates, or any organisations or individuals engaged in lobbying on their behalf.
This site is intended for use only by Australian-registered healthcare professionals with an interest in smoking cessation. It is not intended for use by consumers.
This content is gated for Australian healthcare professionals only
This website is a source of educational content for Australian-registered healthcare professionals only. You must not use the content for any other purpose. If you are a patient seeking medical advice please see your healthcare professional.
By accessing this website you are agreeing that you are an Australian-registered healthcare professional with a current AHPRA registration and are entitled to access this content. Your use of this content is also subject to our Terms of Use and Privacy Policy.